As published in Insight Magazine 2023, Rhianon Bowman
Glaucoma specialist and medical geneticist DR JANEY WIGGS revealed two new genes in juvenile early-onset glaucoma and dissected the value of polygenic risk scores when she delivered the Glaucoma Update Lecture at RANZCO’s 53rd Congress.
Contemporary glaucoma researchers are using a genetic approach to understand the mechanisms of glaucoma. This relies on the premise that the discovery and characterisation of genes that cause or contribute to glaucoma susceptibility will identify the molecular events causing disease and define the mechanisms that underlie disease development.
As Harvard Medical School Professor of Ophthalmology Dr Janey Wiggs explained to the audience at RANZCO’s Congress, using known genes for diagnostic genetic testing and pre symptomatic screening is critical for glaucoma patients to maximise their available sight, but further research is required.
“We’d like to be able to use genetics in the glaucoma clinic to determine people at high risk for disease, be able to identify people at early stages of disease when even conventional therapies are more effective, and to be able to develop therapeutic approaches based on risk,” she said.
Genetic testing can be done for the genes currently known to cause early-onset forms of glaucoma.
“They’re caused by rare mutations with large biological effects and because of that, they occur in families. Traditionally, they were found using linkage analysis. Now, we use more modern techniques to find these genes using exome and genome sequencing, and currently we have 10 genes identified,” Wiggs said.
“There’s a real benefit to doing genetic testing for early-onset glaucoma. Using only genetic information, we can target surveillance and therapy to people in families who have the mutation. We can eliminate unnecessary surveillance of people in these families who don’t have the mutation, which is a great benefit to families,” Wiggs said.
To illustrate her point, Wiggs described the case of a mother-of-four who was diagnosed with glaucoma at approximately 40 years of age (see diagram above). She had surgery to control her intraocular pressure but was worried about her children’s risk. Wiggs’ lab did genetic testing on the mother, and found she carried the myocilin (MYOC) mutation, one of the 10 known early-onset glaucoma genes. They then tested all four children and found that the oldest son had the mutation and also had early evidence of disease as his intraocular pressure was slightly high, but his optic nerves were normal.
“We could initiate a surveillance plan for him and even potentially start treating his pressure since we know he has this mutation. Her younger son also had the mutation but had no evidence of disease, so we could also initiate a surveillance plan for him. Her two daughters did not have mutation or evidence of disease; we could reassure them that their risk of disease development is no greater than that of the average population,” Wiggs said.
While families are appreciative of information about individual disease risk among their children and relatives, it’s not applicable to all families. Currently, the 10 known genes that cause early-onset glaucoma only account for disease in approximately 20% of patients.
“We really need to identify new genes and genetic causality that is responsible for the remaining individuals. Because these genes have been previously identified primarily in US or European Caucasian populations, we hypothesised that if we looked in other populations, we might be able to find additional genes for these early-onset conditions,” Wiggs said.
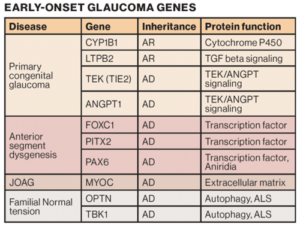
Ten early-onset glaucoma genes have been identified.
EFEMP1
Wiggs’ lab recruited 12 glaucoma-affected families (186 individuals) from the Philippines to test their hypothesis.
“We did whole exome sequencing where we sequence the entire coding region of the genome. We looked for mutations in genes that we already know cause these conditions and we found three families with MYOC mutations and one with a mutation in PAX6,” Wiggs explained.
“Then we applied the sequence data to a bioinformatics pipeline to identify DNA variants that are likely to contribute to disease. Interestingly, three of these families had mutations in a gene called EFEMP1 (also called Fibulin-3). These mutations affect important parts of the protein in terms of function.
They found that mutations that cause severe juvenile glaucoma cause increased intracellular protein retention, and that the extent that the protein is retained is correlated with disease severity.
“This work has led to an EFEMP1 genotype phenotype correlation, where we think there’s a correlation between how much protein is retained intracellularly and how severe the glaucoma is. The more that’s inside the cell, the more severe the disease,” she said.
“EFEMP1 is a new gene for juvenile glaucoma. Importantly, we have not seen these mutations in European Caucasian families. The absence of these mutations in those families suggests that doing this kind of work in other ethnicities and populations is important for detecting new disease-causing mutations.”
Thrombospondin-1 (THBS1)
Studying families with different ethnic backgrounds, Wiggs’ team have also successfully identified Thrombospondin-1 (THBS1), a protein in the ocular anterior segment extracellular matrix, as another gene that causes early-onset glaucoma.
After first identifying a THBS1 mutation in a European Caucasian family in the US, Wiggs reached out to colleagues in the US and Australia, including Professor Jamie Craig at Flinders University, to expand her study.
“Jamie had two additional families that also had mutations in this gene that affected the same protein amino acid, which is an incredible result. We collaborated with a group at Boston Children’s Hospital and made a mutant mouse that had one of these mutations at this amino acid. The mouse also had elevated intraocular pressure, reduced outflow facility and reduced retinal ganglion cells, all consistent with a diagnosis of glaucoma,” Wiggs said.
“Interestingly, the mutant thrombospondin forms protein aggregates in the trabecular meshwork, the structure responsible for draining intraocular fluid. The thrombospondin mutation causes the protein to become unstable and form aggregates in the trabecular meshwork extracellular matrix causing disease. This was also confirmed looking at electron microscopy, showing that the thrombospondin formed these dense deposits in the trabecular meshwork.”
Wiggs said adding two new genes – EFEMP1 and THBS1 – to their repertoire of early-onset glaucoma genes will add to their ability to offer comprehensive genetic testing for affected families.
“It’s interesting too both genes are impacting the extracellular matrix. There are so many other proteins that we know of that function in the extracellular matrix in the trabecular meshwork and that present interesting candidates also for similar phenotypes.”
Adult-onset diseases and polygenic risk scores
One of the most significant problems with populations at risk for glaucoma is that there are many people in the population who have elevated intraocular pressure, but only a fraction of those will go on to develop damage to the optic nerve and be diagnosed with glaucoma, Wiggs said.
“Compounding this issue is that we have some people who develop damage to the optic nerve without ever having elevated intraocular pressure. So, what kind of glaucoma screening tests can we develop that are clinically useful and also cost-effective?”
Wiggs believes the answer is genetics, which is more cost-effective than measuring intraocular pressure combined with OCT, or other measures of the optic nerve function, in glaucoma suspects.
“Unlike early-onset diseases where a single mutation is enough to cause disease, in the adult-onset cases, each individual genetic variant that contributes to disease has a small incremental effect, but in aggregate, the disease threshold can be reached,” she said.
“For the adult-onset diseases, it doesn’t make sense to test a single gene or a single DNA change, but instead to test a population for an array of genetic variants, that in aggregate can influence disease risk.”
This has given rise to the concept of the polygenic risk score (PRS): a measurement of genome-wide genetic risk. Individuals are scored based on the total number of risk alleles that they carry, and then analysed according to the population distribution.
“To develop this kind of opportunity for glaucoma, we – together with Professor David Mackey (Western Australia) and glaucoma genetic leaders around the world – formed the International Glaucoma Genetics Consortium that included all the datasets that were in our large genome-wide association study for primary open angle glaucoma,” Wiggs said.
Associate Professors Stuart MacGregor and Puya Gharahkhani from QIMR Berghofer Medical Research Institute in Brisbane led the genome-wide association study, which identified a total of 127 independent genomic loci.
“This is the kind of genetic data that we need to be able to develop these polygenic risk scores. Using this kind of genome-wide association study result, we can calculate a glaucoma PRS. But how do we know this is going to be effective for glaucoma?” she quizzed.
Craig, MacGregor and colleagues performed a landmark study showing that people who were in the 90th percentile of a glaucoma PRS had a 15-times higher risk of developing glaucoma compared to people at the bottom of the distribution.
“That’s a phenomenal risk effect. They also showed that people in the highest genetic risk profile also develop disease at a much younger age – five to 10 years younger – than people in the lower genetic burden groups. They had thinner nerve fibre layer thickness, so more severe disease, and they also had a much greater need for incisional glaucoma surgery for treatment,” Wiggs said.
“The PRS for primary open angle glaucoma is a tremendous asset for risk stratification. We can find people who are at high genetic risk for earlier onset disease and treat them earlier and potentially more effectively.”
In a separate study, Wiggs’ team showed that patients who have a MYOC mutation – one of the mutations that cause early-onset glaucoma – and have a high genetic risk defined by the PRS, have higher disease prevalence and more severe disease than only having the MYOC gene.
More questions than answers
Despite identifying two new early-onset glaucoma genes and applying polygenic risk stratification for increased surveillance and earlier treatment, Wiggs said more can be done.
“We still need a better understanding of the genetics of early-onset glaucoma. Further gene discovery is important to be able to offer comprehensive genetic testing for individuals at risk,” Wiggs said.
Her team is involved in a large consortium project (ClinGen) to annotate all the known genes that can cause early-onset glaucoma and all the mutations in those genes. In Australia, this is an effort led by Dr Emmanuelle Souzeau at Flinders University.
“ClinGen represents a tremendous opportunity to understand known existing genetic contributions. We’re also involved in a large whole genome sequencing project with Professor Jaimie Craig to identify novel genes that cause these early-onset diseases. We’re also interested in the impact of polygenic risk and polygenic effects on these early-onset glaucoma diseases,” she said.
Wiggs said more work is needed to fully understand the clinical outcomes of polygenic risk for primary open angle glaucoma and other types of adult-onset glaucoma.
“We want to know about the clinical features that are associated with high polygenic risk, what factors can modify the polygenic risk score: people who have high polygenic risk and don’t develop disease – or people who have low polygenic risk and do develop disease – why is that? We also need to understand how polygenic risk affects risk of family members. What is the family risk of somebody who has a high polygenic risk score?,” Wiggs said.